Introduction: The use of standardized, evidence-based classification systems is crucial for the accurate diagnosis and treatment of diseases. Moreover, standardized classification facilitates research and epidemiologic studies and promotes consistency in communication among healthcare professionals. Since 2016, the revised 4 th edition of the World Health Organization classification (WHO-HAEM4R) has been the global standard for diagnosis of lymphoid malignancies. With the emergence of new data, 2 new classification systems were developed and published in 2022: the 5 th edition of the WHO Classification (WHO-HAEM5) and the International Consensus Classification (ICC). Both WHO-HAEM5 and ICC maintain a shared fundamental concept of disease classification that integrates clinical, pathologic, and molecular data. However, they differ on nomenclature, establishment of new entities, and/or diagnostic criteria for some disease categories. To evaluate the impact of these differences on real-world diagnosis of non-Hodgkin lymphoma (NHL), we examined the diagnostic classification of NHL in the Lymphoma Epidemiology of Outcomes (LEO) Cohort Study (NCT02736357).
Methods: Initiated in 2015, LEO is an ongoing prospective observational study of patients aged ≥18 years with newly diagnosed NHL. Patients are enrolled at 8 major U.S. medical centers. Clinical, epidemiologic, pathologic, and treatment data are abstracted at baseline and active follow-up is conducted for all patients. Lymphoma subtype is coded for each case based on expert hematopathology re-review of the diagnostic pathology slides, the pathology report, biomarker data, and clinical data. Of note, NHL subtype distribution is similar to SEER data. We studied all patients enrolled in LEO between 7/1/2016 (the date LEO pathology review started using WHO-HAEM4R diagnostic codes) and 5/31/2020. WHO-HAEM4R diagnoses and additional clinicopathologic data, when relevant, were used to map cases into the corresponding WHO-HAEM5 and ICC diagnoses. Differences between WHO-HAEM5 and ICC were annotated for each case as: None - same disease entity; Minor - difference in nomenclature with similar diagnostic criteria; Major - difference in disease category and/or diagnostic criteria; or Unevaluable - insufficient data to assign WHO-HAEM5 and/or ICC diagnosis.
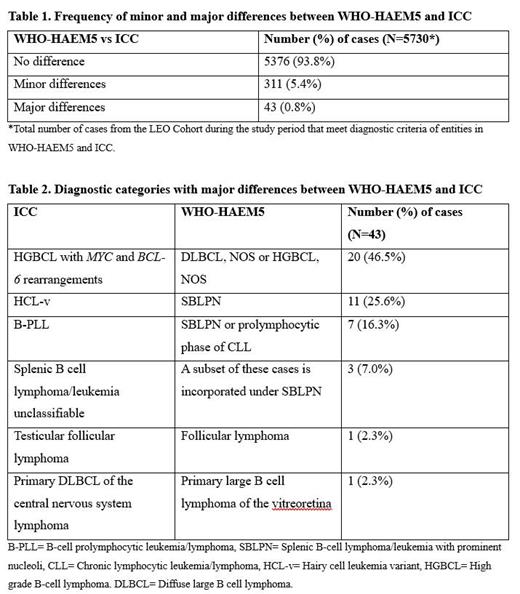
Results: LEO enrolled 6143 patients during the study period. Of these, comparison between WHO-HAEM5 and ICC was evaluable in 5730 (93.3%). Unevaluable cases included those without a specific WHO-HAEM4R diagnosis at enrollment (N=384; e.g., patients with a low-grade B-cell lymphoma that could not be definitively sub-classified) and those with a specific WHO-HAEM4R diagnosis, but with insufficient clinicopathologic data to assign a specific WHO-HAEM5 and/or ICC diagnosis (N=29). Of the 5730 evaluable cases, 5376 (93.8%) showed no difference between WHO-HAEM5 and/or ICC diagnosis, 311 (5.4%) showed minor differences (nomenclature only), and 43 (0.8%) showed major differences (Table 1). The 43 major differences all involved B-cell NHLs; 20 (46.5%) were attributable to different approaches to classifying double-hit lymphomas, 21 (48.9%) to classification of splenic/leukemic B-cell lymphomas, and 2 (4.6%) to classification of B-cell lymphomas occurring at specific anatomic sites (Table 2).
Conclusion: In a large, prospective lymphoma cohort reflecting real-world clinical practice at 8 major U.S. medical centers, major diagnostic differences in the classification of NHL using WHO-HAEM5 or ICC classification criteria were seen in 0.8%, whereas the remaining 99.2% of diagnoses were either the same or showed differences in nomenclature only. The existence of 2 concurrent classification systems presents potential for discrepancies in pathologic diagnosis, clinical practice, clinical trials, and other lymphoma research. Furthermore, some of the differences between WHO-HAEM5 and ICC are clinically and potentially therapeutically significant, and their resolution requires further study. Nevertheless, our findings argue that the proportion of patients affected would be small in real-world practice settings. This appears largely related to incidence rates of specific lymphoma subtypes, with major differences between the 2 classifications predominantly affecting rare entities, while there is general concordance on the most common forms of NHL.
Disclosures
Habermann:sorrento: Research Funding; Genentech: Research Funding; BMS: Research Funding. Cohen:BMS/Celgene: Research Funding; Lam Therapeutics: Research Funding; BioInvent: Research Funding; Genentech: Research Funding; Novartis: Research Funding; Takeda,: Research Funding; ADCT: Consultancy; AstraZeneca: Consultancy, Research Funding; Abbvie: Consultancy; Janssen: Consultancy; BeiGene: Consultancy; Loxo/Lilly: Consultancy, Research Funding. Lossos:NCI: Research Funding; Adaptive: Honoraria; LRF: Membership on an entity's Board of Directors or advisory committees; University of Miami: Current Employment; NCI: Research Funding; BeiGene: Consultancy. Martin:AbbVie, AstraZeneca, Beigene, Epizyme, Genentech, Gilead, Janssen, Pepromene, Daiichi Sankyo: Consultancy. Nastoupil:Gilead Sciences/Kite Pharma: Honoraria, Research Funding; Regeneron: Honoraria; AstraZeneca: Honoraria; Genentech, Inc., Genmab, Gilead/Kite, Janssen, Merck, Novartis, Takeda: Honoraria, Research Funding; AbbVie: Honoraria; Daiichi Sankyo: Honoraria, Research Funding; DeNovo: Honoraria; Caribou Biosciences: Honoraria, Research Funding; Bristol Myers Squibb/Celgene: Honoraria, Research Funding; ADC Therapeutics: Honoraria. Vega:Allogene: Research Funding; Geron: Research Funding. Flowers:Foresight Diagnostics: Consultancy, Current holder of stock options in a privately-held company; Guardant: Research Funding; Nektar: Research Funding; Denovo Biopharma: Consultancy; Genentech Roche: Consultancy, Research Funding; Genmab: Consultancy; Celgene: Consultancy, Research Funding; Allogene: Research Funding; Adaptimmune: Research Funding; Acerta: Research Funding; Amgen: Research Funding; 4D: Research Funding; Sanofi: Research Funding; Pfizer: Research Funding; Novartis: Research Funding; Takeda: Research Funding; TG Therapeutics: Research Funding; Pharmacyclics: Research Funding; Gilead: Consultancy, Research Funding; Karyopharm: Consultancy; Bayer: Consultancy, Research Funding; Eastern Cooperative Oncology Group: Research Funding; Burroghs Wellcome Fund: Research Funding; Ziopharm: Research Funding; Xencor: Research Funding; Morphosys: Research Funding; Kite: Research Funding; N-Power Medicine: Consultancy, Current holder of stock options in a privately-held company; Beigene: Consultancy; Pharmacyclics Jansen: Consultancy; Spectrum: Consultancy; Cellectis: Research Funding; Iovance: Research Funding; Jannsen Pharmaceuticals: Research Funding; SeaGen: Consultancy; Abbvie: Consultancy, Research Funding; National Cancer Institute: Research Funding; V Foundation: Research Funding; Cancer Prevention and Research Institute of Texas: Research Funding; CPRIT Scholar in Cancer Research: Research Funding. Cerhan:Genmab: Research Funding; BMS: Membership on an entity's Board of Directors or advisory committees, Research Funding; Protagonist: Other: Safety Monitoring Committee; NanoString: Research Funding; Genentech: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal